Soil acidity and alkalinity are commonly expressed by pH value, which refers to the degree of acidity and alkalinity of the soil.
Soil acidity and alkalinity are divided into 7 levels, and the grading indexes, pH reflecting intensity are as follows:
-
4.5 Extremely acidic;
-
4.5 - 5.5 strongly acidic;
-
5.5 - 6.5 acidic;
-
6.5 - 7.5 neutral;
-
7.5 - 8.5 alkaline;
-
8.5 - 9.5 strong alkaline;
-
9.5 Extremely alkaline.

Southern red and yellow soils mostly show acidic reaction with pH between 5.0 and 6.5, and individual soils even have pH 4.
In contrast, northern soils are generally neutral or alkaline reactive, with pH values between 7.0 and 8.5.Neutral soil with the highest fertilizer utilization.
Soil acidity and alkalinity identification
1.Determine acidic soil and alkaline soil by soil source
Humus soils in mountain forests, gullies, usually black or brown soils, are relatively loose, fertile, permeable and very good acidic humus. For example: pine needle humus, grass charcoal humus, etc.
2.Determine acidic soil and alkaline soil by surface plants
When collecting soil samples, you can observe the plants growing on the surface. Generally, the soil where pine trees, fir plants and rhododendron grow is mostly acidic; while the soil where cereals, sorghum and halophyllum lots grow is mostly alkaline.
3.Determine acidic soil and alkaline soil by soil color
Acid soils are generally darker in color, mostly dark brown, while alkaline soils are mostly lighter in color, such as white and yellow. In some saline areas, there is often a white layer of saline on the soil surface.


4.Judging acidic soil and alkaline soil by hand feeling
Acid soil in the hands is generally soft, loose soil is easy to spread, not easy to clump; alkaline soil in the hands feel quite hard, easy to clump after release and not spread.

5.Judging acidic soil and alkaline soil by the state after watering
Acid soil after watering faster infiltration, no white bubbles, water surface more muddy; alkaline soil after watering, infiltration slower, water surface white bubbles, white foam, and sometimes the surface of a layer of white alkaline material.
6.Judging acidic soil and alkaline soil by texture
Acid soil is loose in texture and has strong air permeability; alkaline soil is hard in texture and easy to slab into lumps, and the soil is prone to slabbing.
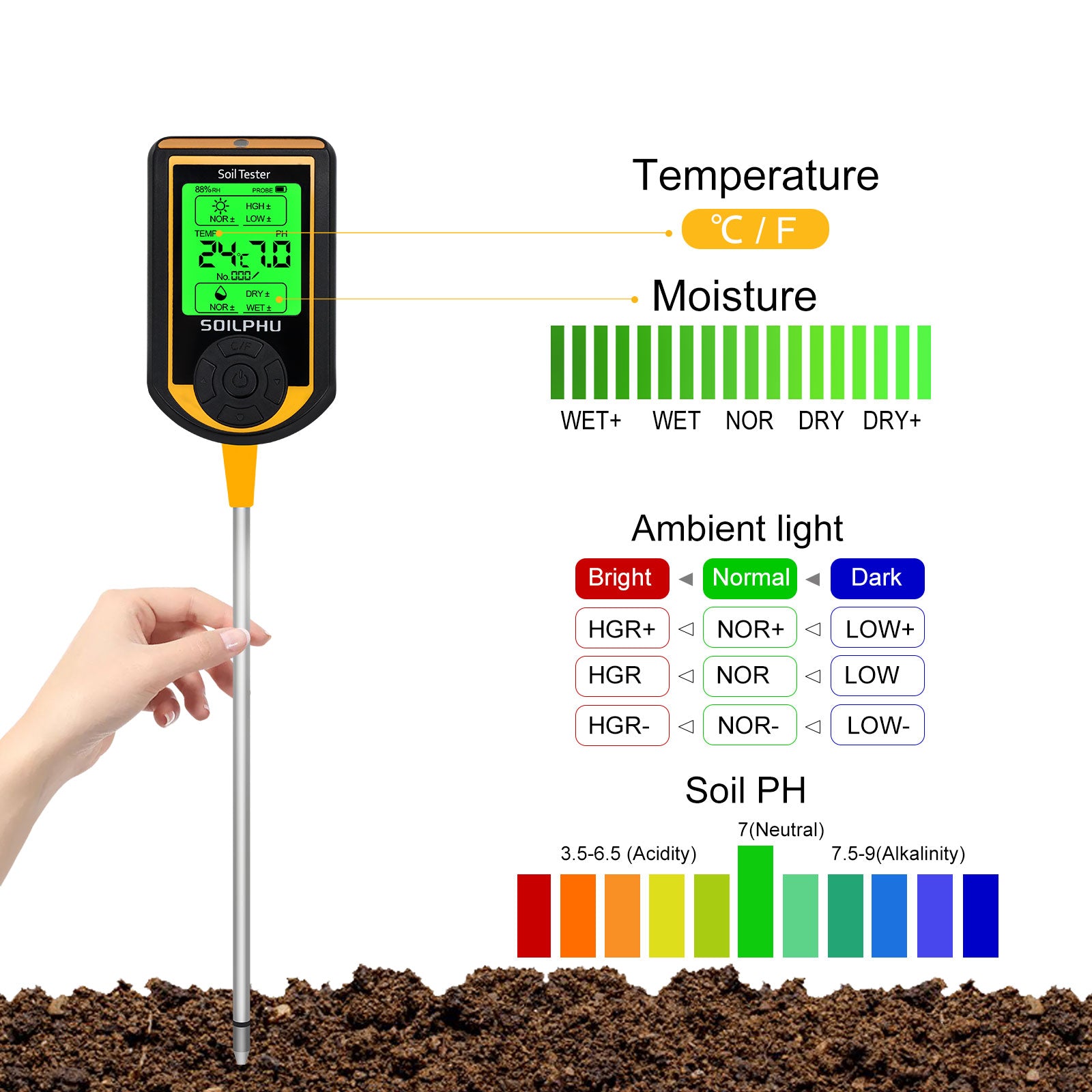
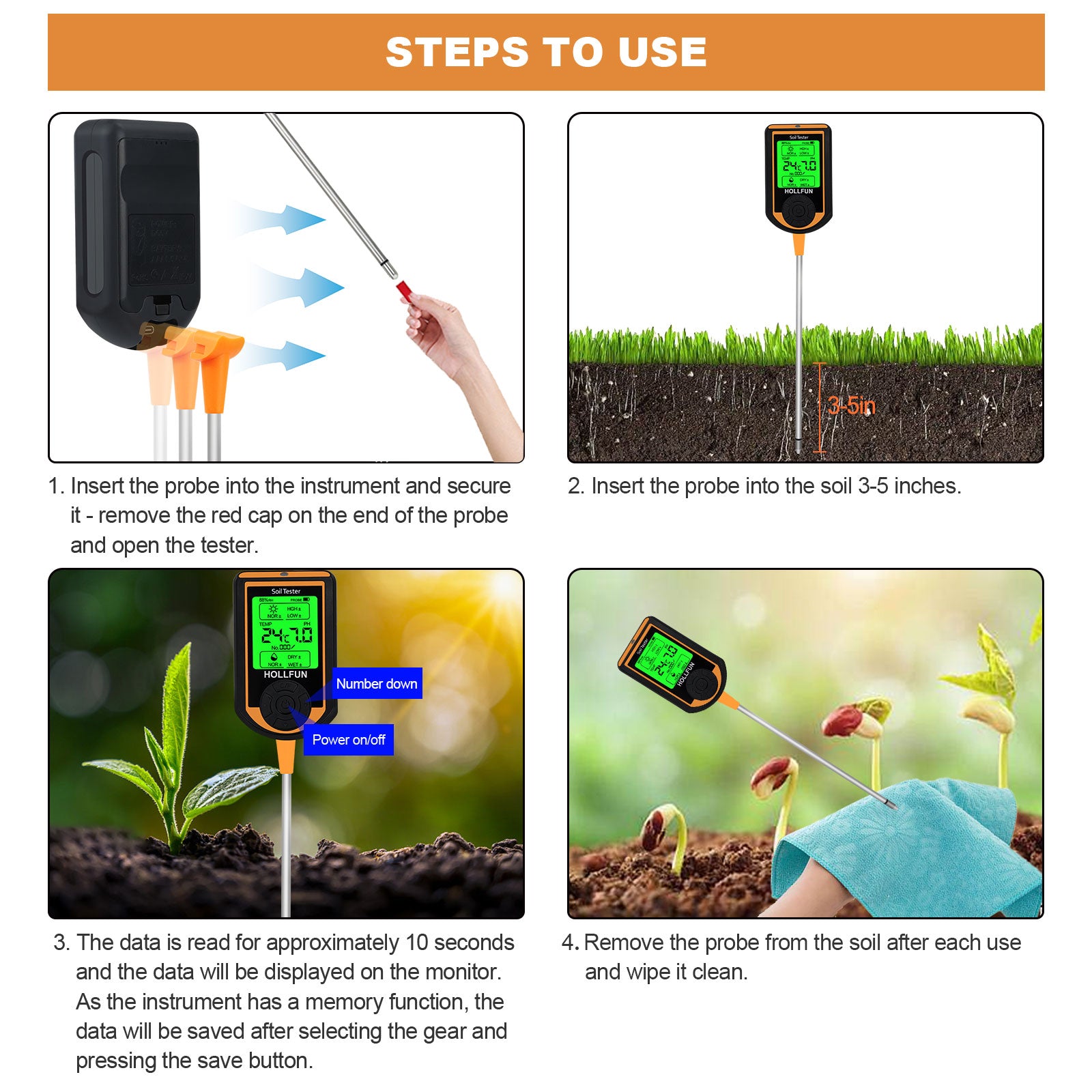
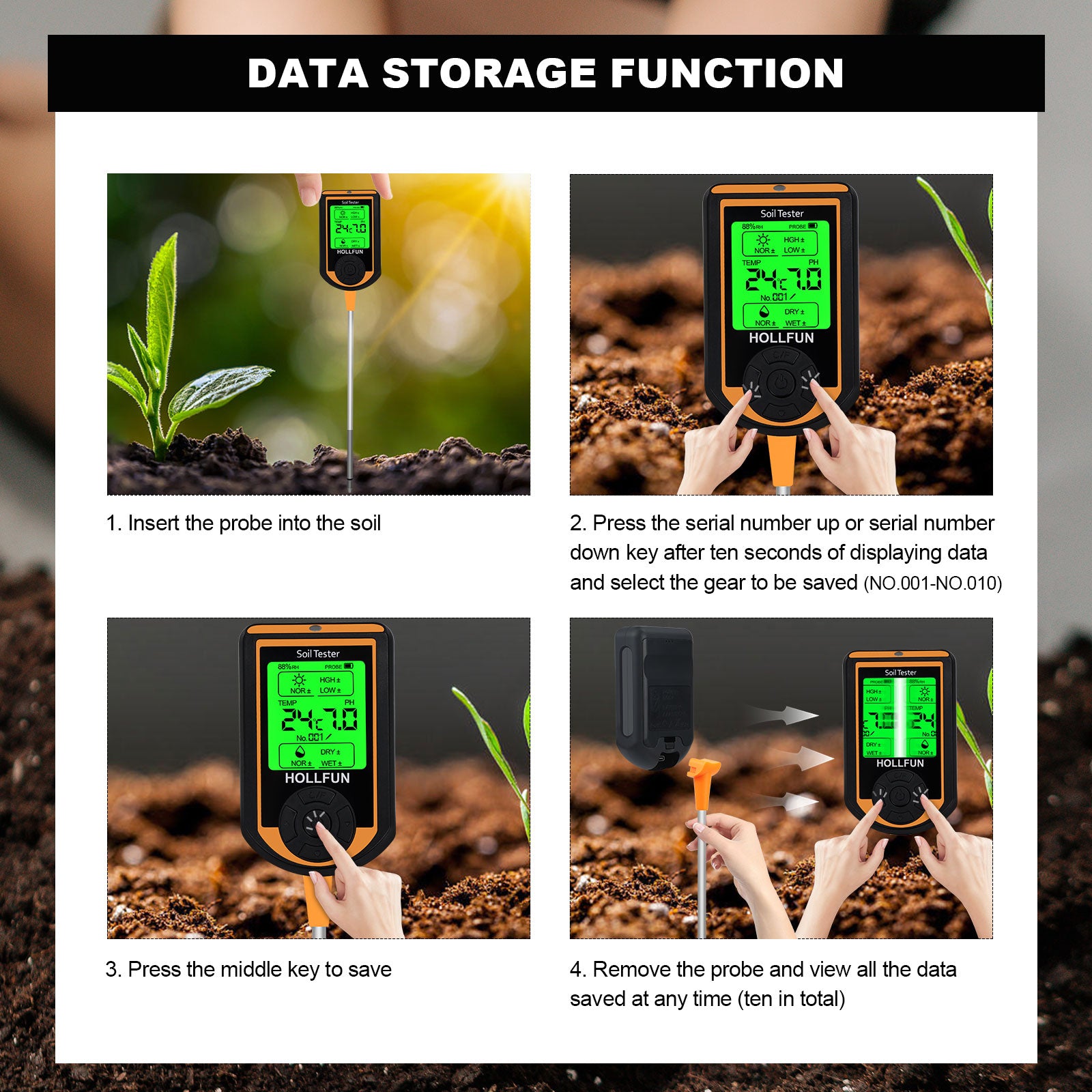
7.Determining acidic and alkaline soils by pH paper or using electronic instruments
Take part of the soil sample and soak it in cool boiling water, immerse part of the test paper in the soaking solution, then take it out and observe the color change, then compare the test paper with the colorimetric card, if pH = 7, the soil is neutral; if pH < small, it is acidic; if pH > 7, it is alkaline.The ph data can also be read using more convenient electronic test instruments.
the impact on plants
1.Most plants have difficulty growing at pH >9.0 or <2.5. Plants can grow normally in a wide range, but various plants have their own suitable pH.
Acid-loving plants: Rhododendron spp., Echinacea spp., Camellia spp., cedar, pine, rubber tree, broomrape;
Calcium-loving plants: alfalfa, grass lucerne, southern aspen, cypress, linden, elm, etc;
Salinity-loving plants: tamarisk, date palm, wolfberry, etc.
2.Plant pests and diseases are directly related to soil acidity and alkalinity:
(1) Subterranean pests often require a range of pH conditions e.g., bamboo locusts like acids while golden turtles like bases;
(2) Some diseases only attack in a certain pH range, such as sudden collapse disease tends to occur on alkaline and neutral soils.
3.Soil activated aluminum:
In strongly acidic soils containing much aluminum, plants living on such soils tend to be aluminum-tolerant or even aluminum-loving (broomstone orchid, tea tree); however, for some plants, such as clover and alfalfa, aluminum is toxic and growth is inhibited when the soil is rich in aluminum; studies have shown that aluminum poisoning is an important cause of ground strength decline in plantation forests.
the impact on nutrients
1.within the normal range, plants are sensitive to soil acidity and alkalinity because soil pH affects the concentration of various ions in the soil solution and the effectiveness of various elements for plants;
2.Effect of soil acidity and alkalinity on the effectiveness of nutrients:
(1) The higher effectiveness of nitrogen at 6-8 is due to the reduced activity of nitrogen-fixing bacteria at less than 6 and the inhibition of nitrification at greater than 8;
(2) Phosphorus is more effective at 6.5-7.5, due to the ease of formation of iron phosphate and aluminum phosphate at less than 6.5 and reduced effectiveness, and the ease of formation of calcium dihydrogen phosphate at higher than 7.5;
(3) Acidic soil leaching is strong, potassium, calcium and magnesium are easily lost, resulting in the lack of these elements. (b) At pH higher than 8.5, soil sodium ions increase and calcium and magnesium ions are replaced to form carbonate precipitates, so the effectiveness of calcium and magnesium is best at pH 6-8;
(4) Five trace elements of iron, manganese, copper, zinc and cobalt are highly effective in acidic soils because they are soluble; molybdate is insoluble in acid but soluble in alkali and is easily deficient in acidic soils; borate is more effective at pH 5-7.5.

Impact on soil fertility
1.Make the effectiveness of soil nutrients decrease.
The effectiveness of phosphorus in soils is clearly influenced by the acidity and alkalinity of At pH values above 7.5 or below 6, phosphoric acid and calcium or iron or aluminum form a late-effective state, making it less effective.
Calcium, magnesium and potassium are easily replaced and leached in acidic soils.
Calcium and magnesium have low solubility and reduced effectiveness in strongly alkaline soils.
Trace elements such as boron, manganese and copper are much less effective in alkaline soils.
Molybdenum precipitates with free iron and aluminum in strongly acidic soils, which can reduce effectiveness.
2.It is not conducive to the benign development of the soil and destroys the soil structure.
Strongly acidic soils and strongly alkaline soils have more H+ and Na+ and lack Ca2+, making it difficult to form a good soil structure and unfavorable to crop growth.
3.unfavorable soil microbial activity.
The general optimum pH for soil microorganisms is a neutral range between 6.5 and 7.5. Too acidic or too alkaline can severely inhibit the activity of soil microorganisms and thus affect the conversion and availability of nitrogen and other nutrients.
4.It is not conducive to the growth and development of crops.
General crops grow best in neutral or near-neutral soils. Sugar beet, alfalfa, red clover is not suitable for acidic soil; tea requires strong acidic and acidic soil, neutral soil is not suitable for growth.

Soil improvement methods:
When cultivating crops, the first thing to find out is the pH suitable range of the crop being cultivated, whether it likes acidic or neutral soil or can be suitable for alkaline soil. If the soil pH is not suitable, it needs to be adjusted and improved.
For overly acidic soil, you can apply 20-25 kg of lime per mu per year, and apply sufficient farmyard manure, avoid only applying lime without farmyard manure, so that the soil will become yellow and thin instead. Apply 1-3 months before sowing to avoid the impact on crop germination and growth. You can also apply 40-50 kg of grass ash to neutralize the soil acidity and better regulate the water and fertilizer condition of the soil.
As for alkaline soil, it is usually improved by applying 30-40 kg of gypsum per mu as a base fertilizer.
When the soil is too alkaline, you can add a small amount of aluminum sulfate (application needs to be supplemented with phosphorus fertilizer), ferrous sulfate (fast-acting, but the effect is not long, need to be applied frequently), sulfur powder (slow-acting, but more durable), highly active humic acid nutrition Carmel Redding, Jinli, etc., the specific amount of application according to the soil pH to determine.
Often pour some diluted water of ferrous sulfate or aluminum sulfate to make the soil more acidic. Humic acid fertilizers can adjust soil pH more safely because they contain more humic substances, such as Carmel Reddit, Winnipeg, and Helicol. Aluminum sulfate is also used to adjust soil pH because it hydrolyzes to produce aluminum hydroxide while producing a small amount of dilute solution of sulfuric acid.
If there are crops growing in the field, additional acidic and alkaline fertilizers can be applied to adjust the soil pH. Using alkaline elements such as calcium and magnesium to replace hydrogen ions raises the pH and also provides nutrients to the crop.

Reasonable application of organic activation nutrient package fertilizer Jiamei Hongli, Winning Lilly, Haili Bao, these fertilizers can directly supplement the crop with organic activation nutrients, saving the time and energy of conversion and significantly improving the buffering performance of the soil, but it is recommended to dissolve in water for use to prevent loss and improve fertilizer efficiency.
Increasing the application of organic fertilizer, which is the most fundamental measure to regulate soil pH, can improve the buffering property of the soil. Soil buffering is closely related to the humus content in the soil, which is mainly derived from organic matter, therefore, heavy application of organic fertilizer must be emphasized in agricultural production.